Standard Enthalpy Of Formation Nh3 . 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq, undissoc). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation of any element in its standard state is zero by definition. N 2 + 3h2 → 2n h3δh = 2 × −46.0 kj mol−1. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The correct option is b +352 kj mol−1.
from www.numerade.com
the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. N 2 + 3h2 → 2n h3δh = 2 × −46.0 kj mol−1. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. the standard enthalpy of formation of any element in its standard state is zero by definition. 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq, undissoc). The correct option is b +352 kj mol−1. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of.
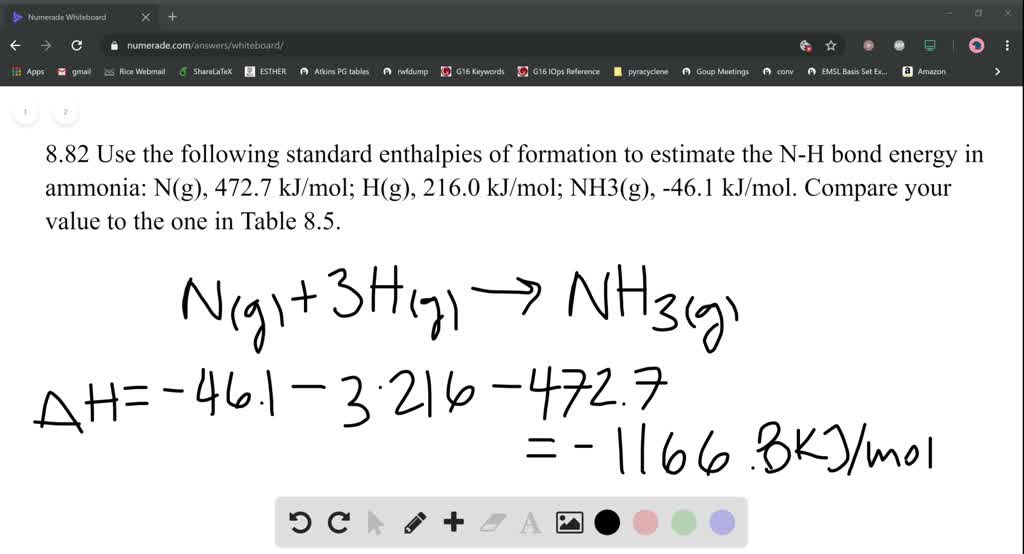
SOLVEDUse the following standard enthalpies of formation to estimate
Standard Enthalpy Of Formation Nh3 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The correct option is b +352 kj mol−1. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq, undissoc). the standard enthalpy of formation of any element in its standard state is zero by definition. N 2 + 3h2 → 2n h3δh = 2 × −46.0 kj mol−1.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJ mol ^1 . If the Standard Enthalpy Of Formation Nh3 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. the standard enthalpy of formation of any element. Standard Enthalpy Of Formation Nh3.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJ mol^1 . If the Standard Enthalpy Of Formation Nh3 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq, undissoc). the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created.. Standard Enthalpy Of Formation Nh3.
From www.toppr.com
N2(g) + 3H2(g) → 2NH3(g); A,Hº = 92.4 kJ mol What is the standard Standard Enthalpy Of Formation Nh3 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq,. Standard Enthalpy Of Formation Nh3.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJ mol. If the Standard Enthalpy Of Formation Nh3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 14 rows the. Standard Enthalpy Of Formation Nh3.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJ mol^1 . If the Standard Enthalpy Of Formation Nh3 The correct option is b +352 kj mol−1. the standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen. Standard Enthalpy Of Formation Nh3.
From askfilo.com
What is the standard enthalpy of formation of NH3 gas, standard heat of Standard Enthalpy Of Formation Nh3 the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. N 2 + 3h2 → 2n h3δh = 2 ×. Standard Enthalpy Of Formation Nh3.
From www.toppr.com
The enthalpy of formation of ammonia is −46.0 kJ mol−1. The enthalpy Standard Enthalpy Of Formation Nh3 The correct option is b +352 kj mol−1. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic. Standard Enthalpy Of Formation Nh3.
From www.numerade.com
SOLVED ( Question 2 What is the standard enthalpy of formation for Standard Enthalpy Of Formation Nh3 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq,. Standard Enthalpy Of Formation Nh3.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Nh3 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq, undissoc). For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Of Formation Nh3.
From www.numerade.com
SOLVEDUse the following standard enthalpies of formation to estimate Standard Enthalpy Of Formation Nh3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. The correct option is b +352 kj mol−1. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. . Standard Enthalpy Of Formation Nh3.
From www.numerade.com
SOLVED According to the information above what is the standard Standard Enthalpy Of Formation Nh3 The correct option is b +352 kj mol−1. 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq, undissoc). the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Standard Enthalpy Of Formation Nh3.
From zavierfersprince.blogspot.com
Ammonia Nh3 Reacts With Hydrogen Chloride to Form Ammonium Chloride Standard Enthalpy Of Formation Nh3 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq, undissoc). the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Enthalpy Of Formation Nh3.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby Standard Enthalpy Of Formation Nh3 the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ),. Standard Enthalpy Of Formation Nh3.
From priaxon.com
What Is Standard Enthalpy Of Formation Of Nh3 Gas Templates Printable Standard Enthalpy Of Formation Nh3 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. N 2 + 3h2 → 2n h3δh = 2 × −46.0 kj mol−1. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy. Standard Enthalpy Of Formation Nh3.
From askfilo.com
The enthalpy of formation of ammonia is =46.0 kJ mol−1. The enthalpy chan.. Standard Enthalpy Of Formation Nh3 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq, undissoc). N 2 + 3h2 → 2n h3δh = 2 × −46.0 kj mol−1. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation of any element. Standard Enthalpy Of Formation Nh3.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJmo17. If the Standard Enthalpy Of Formation Nh3 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq, undissoc). N 2 + 3h2 → 2n h3δh = 2 × −46.0 kj mol−1. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form. Standard Enthalpy Of Formation Nh3.
From kunduz.com
[ANSWERED] 1 The standard enthalpy of formation of NH3 is 46 0 kJ mol Standard Enthalpy Of Formation Nh3 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation of any element in its standard state is zero by definition. N 2 + 3h2 → 2n h3δh = 2 × −46.0 kj mol−1. The correct option is b +352 kj mol−1. 14 rows the. Standard Enthalpy Of Formation Nh3.
From www.numerade.com
SOLVED Predict the standard enthalpy change for the reaction below Standard Enthalpy Of Formation Nh3 14 rows the 13 contributors listed below account for 90.6% of the provenance of δfh° of nh3 (aq, undissoc). N 2 + 3h2 → 2n h3δh = 2 × −46.0 kj mol−1. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The correct option is. Standard Enthalpy Of Formation Nh3.